THE VAGINAL MICROFLORA
Although less complex than the gastrointestinal microflora, the normal vaginal microflora of a premenopausal woman is composed of a variety of bacterial species. Anaerobes are most frequently isolated and appear in numbers of 107 - 109 CFU/ml of vaginal secretion. Lactobacillus spp. is the most frequently isolated genus found in the highest numbers. They play a role in maintaining the balance of the normal vaginal flora by producing hydrogen peroxide. It has been shown that approximately 70% of premenopausal, healthy women harbor hydrogen peroxide-producing lactobacilli. Corynebacterium, Staphylococcus and Bacteroides spp. are among the anaerobes frequently isolated.
INTESTINAL MICROFLORA IN THE NEWBORN INFANT
Fetuses are sterile in the womb, but beginning with the birth process, infants are exposed to microbes that originate from the mother and the surrounding environment including breast milk or formula(12). The infant tends to acquire the flora swallowed from the vaginal fluid at the time of delivery. Because vaginal flora and intestinal flora are similar, an infant's flora may closely mimic the intestinal flora of the mother(15).
Another factor affecting the intestinal flora of the newborn is delivery mode. A normal vaginal delivery commonly permits transfer of bacteria from the mother to the infant. During cesarean deliveries, this transfer is completely absent. These infants commonly acquire and are colonized with flora from the hospital's environment and, therefore, their flora may differ from maternal flora. Infants delivered by cesarean section are colonized with more anaerobic bacteria, especially Bacteroides, than vaginally delivered infants. Clostridium perfringens is the anaerobic bacterium most frequently isolated after cesarean deliveries. When colonized, cesarean delivered infants less frequently harbor E. coli, and more often klebsiella and enterobacteria(7). 
Above Figure: Factors shaping the neonatal microbiome. Maternal vaginal infections or periodontitis can result in bacteria invading the uterine environment. Gut and oral microbiota could be transported through the bloodstream from the mother to the fetus. Delivery mode shapes the initial bacterial inoculum of the newborn. Postnatal factors such as antibiotic use, diet (such as breast-feeding versus formula, and introduction of solid food), genetics of the infant and environmental exposure further configure the microbiome during early life. As diet diversifies with age, the microbiome gradually shifts toward an adult-like configuration, which is usually reached by age 3. Bacteria associated with the different processes are indicated.
Ref.: "The microbiome in early life: implications for health outcomes." Tamburini S, et all; Nat Med. 2016 Jul 7;22(7):713-22.
The initial colonizing bacteria vary with the food source of the infant. In breast-fed infants, Bifidobacteria account for more than 90% of the total intestinal bacteria. The low concentration of protein in human milk, the presence of specific anti-infective proteins such as immunoglobulin A, lactoferrin, lysozyme, and oligosacharides (prebiotics), as well as production of lactic acid, cause an acid milieu and are the main reasons for its bifidogenic charachtersitics. In bottle-fed infants, Bifidobacteria are not predominant(13). Instead enterobacteria and gram-negative organisms dominate because of a more alkaline milieu and the absence of the prebiotic modulatory factors present in breast milk.
The establishment of an intestinal microbial ecology is very variable at the beginning but will become a more stable system similar to the adult microflora by the end of the breastfeeding period.
Other factors affecting the intestinal microflora of the infant include geographical differences (industrialized vs. developing countries) and administration of antibiotics in neonatal intensive care.
EFFECTS OF PROBIOTICS ON THE INTESTINAL MICROFLORA
Probiotics modulate the composition of the intestinal microflora. The survival of ingested probiotics in different parts of the gastrointestinal tract varies. As a result of their concentration in the lumen, they contribute to transient modulation of the microflora ecology, at least during the period of intake. This specific change may be seen for a few days after the start of consumption of the probiotic preparation, depending on the capacity and dosage of the strain in question to modulate the functioning of the gastrointestinal tract. Results show that with regular consumption, the bacteria temporarily colonise the lower intestine. Once consumption stops, the number of probiotic microorganisms quickly falls (see Figure 2 below). This applies to all probiotic supplements available in the market today.
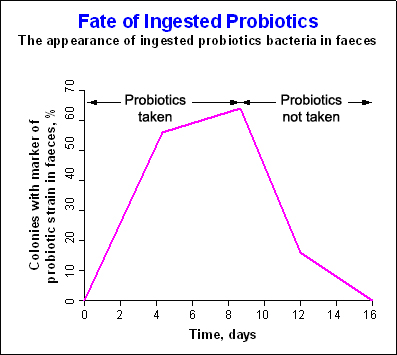
Figure 2
Many studies have demonstrated significant shifts in bacterial counts in human faeces following consumption of specific probiotic strains, generally resulting in increased numbers of health-promoting genera (Lactobacillus and Bifidobacterium) and decreased numbers of potentially harmful ones (such as several strains of Clostridum, Enterococcus and Candida). These studies, however, reflect the bacteriological situation in faecal matter only and do not provide an accurate picture of the situation in different parts of the gastrointestinal tract or in the mucosal layer of the gut. Furthermore, many species of intestinal bacteria from faecal samples cannot be cultured on specific plates. Probiotic bacteria modulate the metabolic activity of the gut flora. Probiotics, being able to lower the pH in the intestinal tract, may thus be able to interfere with the enzymatic activity of the flora.
